The news reached Sarah Gilbert on Saturday night that the COVID-19 vaccine she developed with AstraZeneca seemed to work, but the Oxford University professor hoped for a key number: Was it more than 90 percent effective, as have been others, or less?
Instead, when his colleague Andrew Pollard called with the results, he wanted to show him slides instead of simple figures . “I really didn’t understand why we had to go through slides,” he recalled. “But then it became clear: because it is much more complicated in our test.”
That complexity has led to uncertainty surrounding one of the pioneers along with Pfizer, BioNTech and Moderna in the ‘race’ for a dose to end the pandemic. Questions about the most effective dose of the vaccine, its safety record, and partners’ approach to testing it have cast doubt on whether the US Food and Drug Administration (FDA) will endorse it.
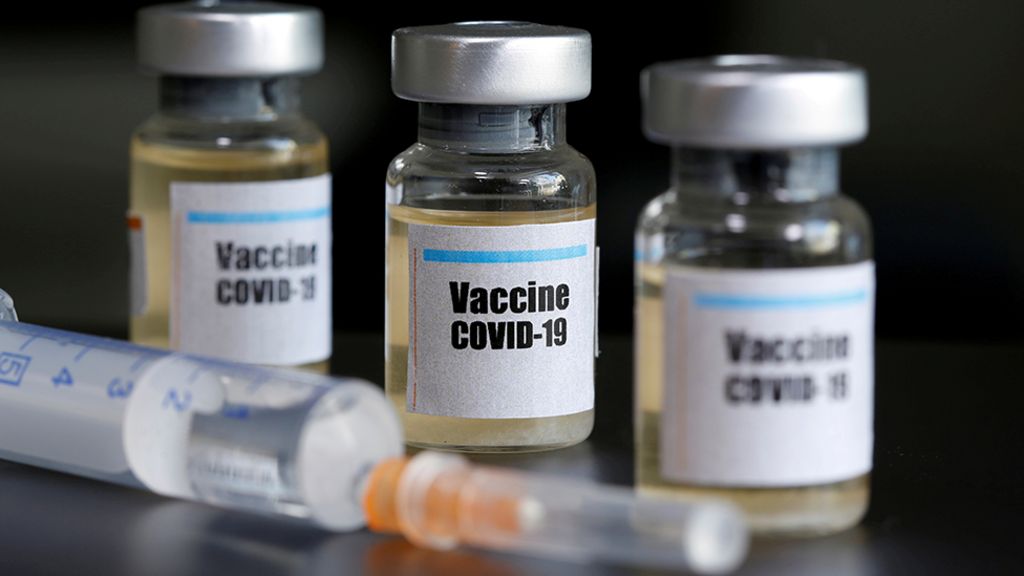
AstraZeneca and Oxford reported the results early Monday after 131 trial participants in the UK and Brazil contracted COVID-19. The average prevention efficacy of 70 percent sounded good , within analyst expectations, and above the 50 percent standard the FDA had set for COVID vaccines.
The study also found 16 serious cases, all among people who received vaccine from AstraZeneca , according to Moncef Slaoui, the exinvestigador of GlaxoSmithKline who runs the program Operation Warp Speed US. It was good news for Astra’s vaccine program that had been on hold for six weeks in that country while regulators reviewed a serious adverse event that the company never gave details about. Oxford will report the details of these adverse events in its efficacy study which it will present for review in the coming weeks.
Shares of AstraZeneca rose as much as 2.4 percent early Tuesday morning in London after falling 3.8 percent on Monday.
The puzzling part of Astra’s results was that the larger doses were less effective. The vaccine was only 62 percent effective in a group that received two full doses about a month apart, but among about 2,700 people who received a half dose followed by a full one , the effectiveness rose to 90 percent.
Positive data from another vaccine of substantial efficacy with potential advantages in storage, transportation and affordability will be welcome , said Jesse Goodman, a former chief of the FDA’s office of vaccines who later became the agency’s chief scientist.
“What is not yet clear is whether this effect is a chance observation or reflects that the higher dose can negatively affect the immune response ,” he said in an interview. “We need more details to begin to understand it.”
Geoffrey Porges, an analyst at SVB Leerink, was among the most critical voices, calling the data “premature and insufficient” in a note to clients and predicting that the vaccine would “never be licensed in the US.”
Arm yourself with patience
AstraZeneca and Oxford are conducting trials of the vaccine in different parts of the world , and are currently only studying two full doses of the dose in their US study, which is expected to include 30,000 volunteers. But the company is still recruiting participants and could add another analysis for further studies of the half-dose regimen, explained Ruud Dobber, executive vice president and president of biopharmaceuticals at AstraZeneca.
“Let’s be a little more patient and see how the FDA reacts before making such harsh statements,” he said in an interview with Bloomberg TV .
The UK Medicines and Healthcare Products Regulatory Agency has already started its analysis based on the data it has received in ongoing review, Chief Executive June Raine said in a statement.
It may interest you: Why the AstraZeneca vaccine, which will be packaged in Mexico, may be the best option?
Low- and middle-income nations are looking to the injection, priced much lower than Pfizer and Moderna, as a way out of the pandemic. The lower efficacy number of the two full-dose regimens reached the World Health Organization benchmark, according to chief scientist Soumya Swaminathan.
Still, Swaminathan warned in an interview Tuesday with Bloomberg Television that more comprehensive data is needed to understand possible discrepancies between doses.
“We really need to wait for bigger numbers, longer follow-up, to see if these differences really continue into the future,” he said.
That a lower dose produced a better result left even Gilbert scratching his head . The researchers tested a starting half-dose regimen to find the smallest amount that could still elicit a strong immune response , Gilbert said, not to increase effectiveness.
“I was surprised,” he said in an interview. “I really didn’t expect that.”
Its superiority over two full doses could be good news, as it would allow the company to perform more inoculations with the same total volume of vaccine . That could be important with the need to vaccinate billions of people around the world.
The vaccine uses a harmless chimpanzee adenovirus , the cause of some common colds, as a vector that is then inserted with the coronavirus spike protein to elicit an immune response.
What could have caused these results?
In theory, it is possible that a full initial dose raised antibodies against the adenovirus vector itself, which could have limited the immune response to the peak protein of the SARS-CoV-2 virus, Gilbert said, but his team measured antibodies against it. adenovirus in previous studies and found only a small effect.
“I’m not really sure if that’s the complete answer. We’ll look into it a bit more, but it may be a bit more subtle in terms of inducing a high-quality immune response by giving just the right amount of vaccine antigen in the first dose and then expanding it with the second dose, “he added.
One possible explanation for the variable results is that the lower starting dose does a better job of breaking through the body’s defenses , allowing the vaccine to infect cells and create the immune response, said Michael Kinch, an expert in drug development and Associate Vice Chancellor at Washington University in St. Louis.
Another theoretical reason is more complex: the immune system can become immune to the initial high dose of vaccine and then ignore the subsequent dose, a phenomenon known as immune tolerance or desensitization. Either one would have the potential to mitigate the effects of the vaccine.
“The 90 percent number may ultimately prove correct,” Kinch said, “but the dose-response reversal could be a sign of concern.”
The Oxford team is still analyzing their data to better understand how the vaccine works. Gilbert noted that he is confident that the higher efficiency number will stick around as more data arrives.
“It could go up or down a bit,” he said. “I don’t think it’s going to change massively, but it may differ from that initial 90 percent in either direction when we get to a little bigger analysis.”


 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  Solana
Solana  USDC
USDC  TRON
TRON  Cardano
Cardano  Lido Staked Ether
Lido Staked Ether  Avalanche
Avalanche  Toncoin
Toncoin