Atoms, Protons, Neutrons, Electrons, Atomic Numbers, Atomic Masses, the Periodic Table, and the list goes on. It seems exciting when we get introduced to chemistry. New concepts come our way, and trying to ace them becomes one of our interests. One of such concepts that make us invest our time is the periodic table and the concepts. Memorising the elements of the Periodic Table is just for fun but essential for your grades too.
It all starts with learning the first 30 elements; we all try to memorise those elements in different ways that suit us. So, here are the top tips for memorising the
Atomic Mass of the First 30 Elements:
You must try this out if you haven’t experimented with other tricks yet.
Hydrogen – H – Happy
Helium – He – Henry
Lithium – Li – Lives
Beryllium – Be – Beside
Boron – B – Boron
Carbon – C – Cottage
Nitrogen – N – Near
Oxygen – O – Our
Fluorine – F – Friend
Neon – Ne – Nelly
Sodium – Na – Nancy
Magnesium – Mg – Mg
Aluminium – Al – Allen
Silicon – Si – Silly
Phosphorus – P – Patrick
Sulphur – S – Stays
Chlorine – Cl – Close
Argon – Ar – Arthur
Potassium – K – Kept
Calcium – Ca – Carrie
This was a simple way in the form of a sentence/phrase to learn the first 20 elements.
Now let’s talk about the top 5 methods to memorise the periodic table. Eventually, atomic masses make their way along post-learning of the elements.
- Visual Memory
Our brains love pictures, and that makes our visual memory techniques 10x more potent than other methods.
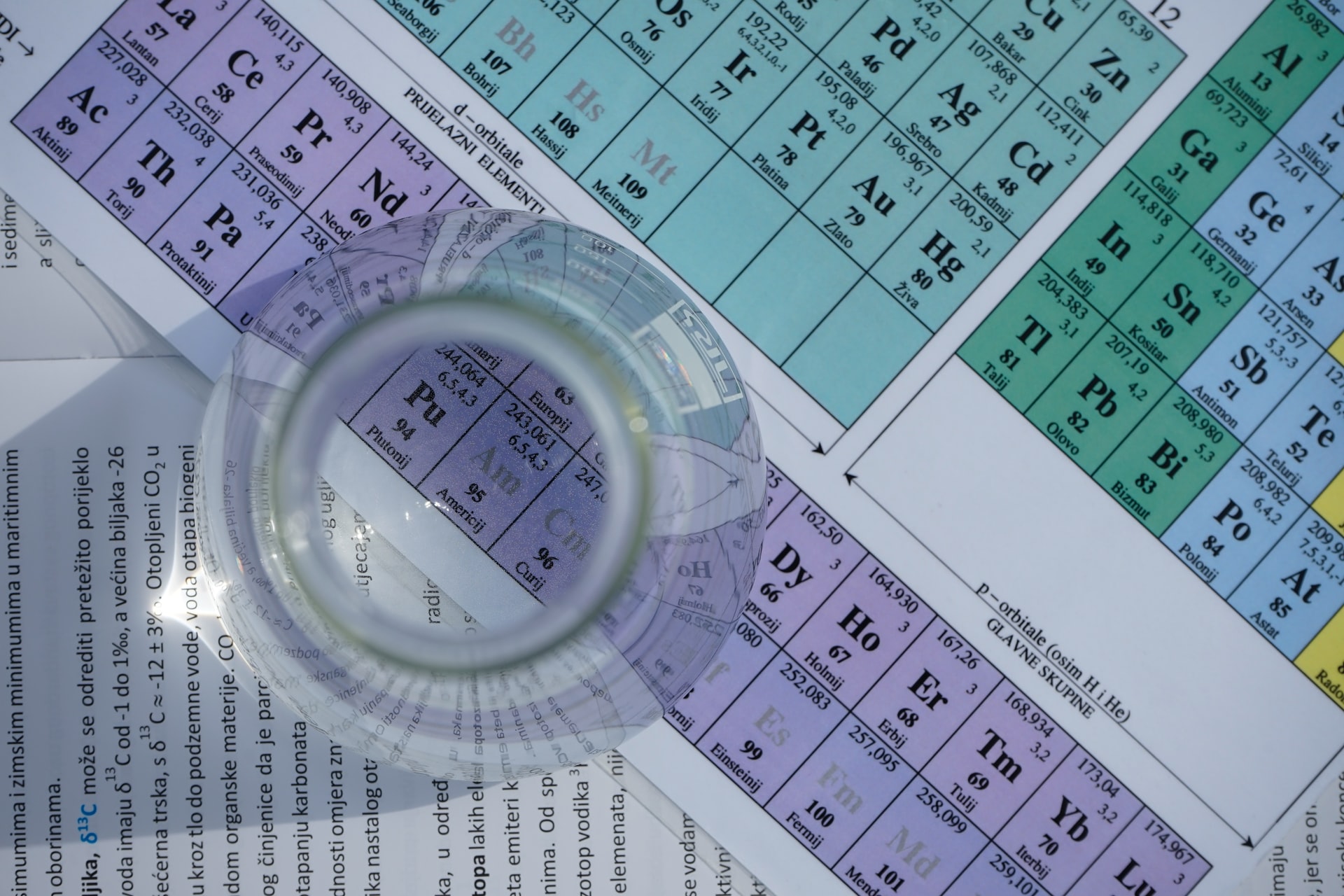
Tip: Get yourself a medium-sized chart of the periodic table. Seeing the picture now and then would help you learn the values faster and easier.
- Sing Self Composed Songs
It is undoubtedly a great way to make repetition fun. Songs do make a way into your brains than any concepts that you try to mug up.
Tip: Create a song that suits you to memorise the elements and let them play in your mind. You can even sing it out loud to help your friends too.
- Acronyms
First letters cues don’t prompt enough to recall the complete element name. But this is a great trick to learn the first 30 as no one asks you to memorise the whole table at once.
Tip: The first letter of each element can be very easy to learn in the table as they are represented similarly.
- Flashcards
This is a great way to recall your memory and practice the concepts now and then. It is an active and convenient way to use repetition. You might want to find shortcuts here, but oops! No shortcuts here.
Tip: Make flashcards for the elements with their atomic number and atomic masses to check your memory. Exciting games will be lined up if you get started with the tricks.
- Try out different Memorising strategies
Repetition is one of the strategies used by students to learn complex concepts. However, it has also been proven to be beneficial for many so that you can try it out too.
Tip: Space out your studying and repetition over several days, and try to increase the time between your study sessions.
The atomic mass of elements of the periodic table is measured with the help of unified atomic mass units.
You need to figure out your tricks and tips to figure out a way of remembering atomic masses. But this method can help you remember the Atomic Mass of the First 30 Elements easily.
- For starters, the easiest and the most used trick to remember the atomic masses is to multiply the atomic number by 2.
The formula behind the atomic masses of elements like He, Li, C, N, O, Ne, Si, S, Cl, Ca are double the atomic number.
For Example:
He = 2 x2 = 4
Li = 3 x2 = 6
C = 6 x2 = 12
- The atomic masses for the rest of the elements like Be, B, F, Na, Mg, Al, P, Ar, and K are not double the atomic number.
For these elements just double the atomic number and add 1
For Example:
Be = 4 x2 1 = 9
Na = 11 x2 1 = 23
Cl = 17 x2 1 = 35
This would not give you the actual atomic masses but values pretty close to the actual data. And for the school-level calculations, this trick would work pretty well. This method works well only for the first 30 (Zn) elements. However, for the later elements that are in the row, the technique becomes inaccurate.
This is a must-try trick to memorise the values faster.
Below are the atomic masses of the first 30 elements. Please take a look and select a trick that suits you better to ace it!
| ATOMIC NUMBER | ELEMENT | ATOMIC MASS |
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.180 |
| 11 | Sodium | 22.990 |
| 12 | Magnesium | 24.305 |
| 13 | Aluminium | 26.982 |
| 14 | Silicon | 28.085 |
| 15 | Phosphorus | 30.974 |
| 16 | Sulfur | 32.06 |
| 17 | Chlorine | 35.45 |
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
| 21 | Scandium | 44.956 |
| 22 | Titanium | 47.867 |
| 23 | Vanadium | 50.942 |
| 24 | Chromium | 51.996 |
| 25 | Manganese | 54.938 |
| 26 | Iron | 55.845 |
| 27 | Cobalt | 58.933 |
| 28 | Nickel | 58.693 |
| 29 | Copper | 63.546 |
| 30 | Zinc | 65.38 |
Simple memory tips and tricks:
- Try and understand the information first.
- Try to link it with the ideas you are familiar with.
- Sleep on it.
- A self-test is a must.
- Write it out.
- Talk to yourself. Explain what you learned.
- Exercise. Practice your techniques.
Final Thoughts
Initially, some of these techniques can feel strange and take time to develop. But as time passes, you will get the hang of it, and the methods will feel natural. You don’t have to try them all; experiment with a few, see what works the best for you, and stick to it.
Hope you found this article helpful. Drop your questions in the comment box, and we would be happy to clarify your doubts.


 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  Solana
Solana  USDC
USDC  TRON
TRON  Cardano
Cardano  Lido Staked Ether
Lido Staked Ether  Avalanche
Avalanche  Toncoin
Toncoin